Vaccines: A Quick Review
Vaccination has proven to be the greatest boon to human and animal health over the past few decades. It is a discovery which has made the largest impact on global health, apart from the introduction of sanitization & clean water, especially in developing countries. The mortality rate used to be massive in the pre-vaccination period (e.g. From smallpox and measles) with up to a half of the population dying during epidemics, but thanks to the development of vaccines there has been a drastic decline in mortality. Major infections like smallpox and rinderpest, have been eradicated since the introduction of vaccination.
The WHO’s immunization programme in 1974 & the global alliance of vaccination in 2000 enhanced global coverage of vaccination against many important infectious diseases. For example, Polio has nearly been eradicated (India & other Southeast Asian nations declared polio-free in 2014) thanks to inactivated polio vaccine (IPV).
Types Of Vaccines
Vaccines are of different types. Each type is created to boost the immune system in fighting different kinds of pathogens and sometimes, the life-threatening diseases they cause.
While developing vaccines, scientists consider various factors such as –
- Which components of the immune system respond to those particular pathogens
- Who needs to be vaccinated
- Technology or approaches to create the vaccine.
Scientists decide which type of vaccine to create based on these factors. Based on these factors, vaccines can be of the following types.
Inactivated vaccines
Inactivated vaccines using the killed version of the pathogen that causes the disease.
These protect against diseases like –
-
-
- Hepatitis A
- Flu (shot only)
- Polio (shot only)
- Rabies
-
Polysaccharide, recombinant, conjugate & Subunit vaccines
Polysaccharide, recombinant, conjugates & Subunit vaccines use specific pieces of the pathogen — like their capsid (protein shell around the pathogen), sugar, or proteins. This type of vaccines gives a very strong immune response as they use only specific pieces of the pathogen.
These vaccines are commonly used in protection against diseases like –
- Hib (Haemophilus influenza type b) disease
- Hepatitis B
- HPV (Human papillomavirus)
- Pneumococcal disease
- Meningococcal disease
- Shingles
Toxoid vaccines
Toxoid vaccines use a toxin made by the pathogen that causes a disease. They create immunity to only those parts of the pathogen that cause disease instead of the pathogens itself. That means the immune response is targeted to the toxin instead of the whole pathogens.
Toxoid vaccines are used to protect against:
- Diphtheria
- Tetanus
How Are They Made?
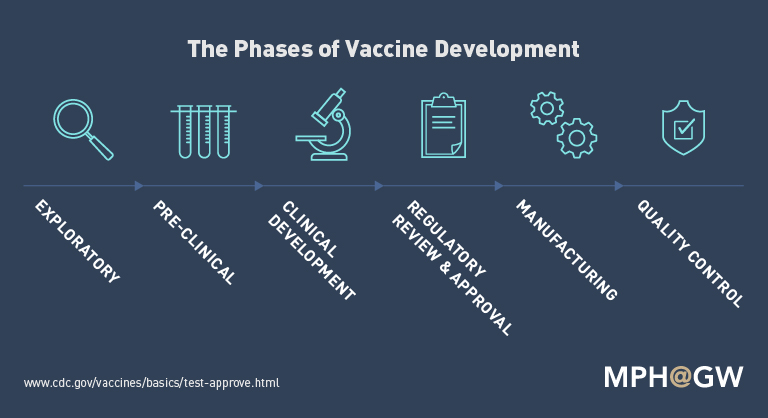
Image source: https://publichealthonline.gwu.edu/blog/producing-prevention-the-complex-development-of-vaccines/
Vaccines are made using the disease-causing virus or bacteria, but in a form that will not harm a person. Instead, the modified or weakened pathogen prompts the immune system to develop antibodies against the disease. Once it is determined how the virus and bacteria will be modified, vaccines are created through the following process.
The first step is the generation of the antigen used to induce an immune response. Steps include the growth and harvesting of the pathogen itself (For later inactivation or isolation of subunit) or generation of a recombination protein (a protein made with DNA technology) derived from the pathogen. Recombination proteins can be manufactured in cultures of bacterial cells or yeast. Viruses are grown in cell cultures. Bacterial pathogens are grown in devices using a growth medium developed to optimize the yield of the antigen while marinating its integrity.
The second step is to release the antigen from the cells and isolate it from the material used for its growth. Proteins and other parts of the growth medium may still be present and must be removed in the next step. The goal of this stage is to release as much as virus or bacteria as possible.
The third step is the purification of the antigen. For vaccines that are made from recombination proteins, this may involve chromatography (a method of separating materials) and ultrafiltration may occur.
The fourth step may be the addition of an adjuvant, which is a material that enhances the strength of the immune response. For a prolonged shelf-life, vaccines may also include stabilizers or preservatives to allow multi-dose vials to be used safely
The final step combines all components that make up the final vaccine and uniformly mix them in a single vessel. Then, the vaccine is filled into vial or syringes packages, sealed with sterile stoppers or plungers, and labelled for widespread distribution. Some vaccines are freeze-dried and then rehydrated at the time of administration.
Vaccines undergo rigorous safety & efficacy testing prior to approval from FDA’s and are continually monitored for their safety. Commercial production and marketing of vaccines involve conducting high-quality manufacturer funded trials before it can be launched & administrated safely to the general public.

Photo by Polina Tankilevitch from Pexels
Vaccines save 2 to 3 million lives every year according to the World Health Organisation. They are the safest and most effective way of protecting us against diseases. But despite these successes, approximately 6.6 million children still die each year and about half of these deaths are caused by infections, including pneumonia and diarrhoea, which could be prevented by vaccination. Success against these infections will require a combination vaccination approach, each module targeting a different part of the immune system. Besides prevention, vaccination can also be used to adjust the course of non-infectious diseases. Scientists have already made progress with therapeutic cancer vaccines and the upcoming potential area includes addiction, diabetes, hypertension and Alzheimer’s disease.
In today’s time, when COVID-19 has wreaked havoc globally & caused around 250k deaths till now, everyone is praying for a miracle vaccine. Many pharmaceutical companies and institutes are working rigorously to develop a vaccine. Few of them like serum institute of India, Pfizer & GSK have already started clinical and may be able to create vaccines which will be available for distribution by 2021.